Ensure You’re Ready for the New MoCRA Requirements
The Modernization of Cosmetics Regulation Act significantly expands the authority of the Food and Drug Administration (FDA) to oversee the industry and enforce higher standards in documentation and product safety.
That means more change is in store for your cosmetics company, and both due diligence and thorough preparations for the new requirements are in order. Failure to do so could result in steep consequences for your organization for any violations of the MoCRA regulations. These may include costly mandatory product recalls, class-action lawsuits, penalty fees and even complete business closure.
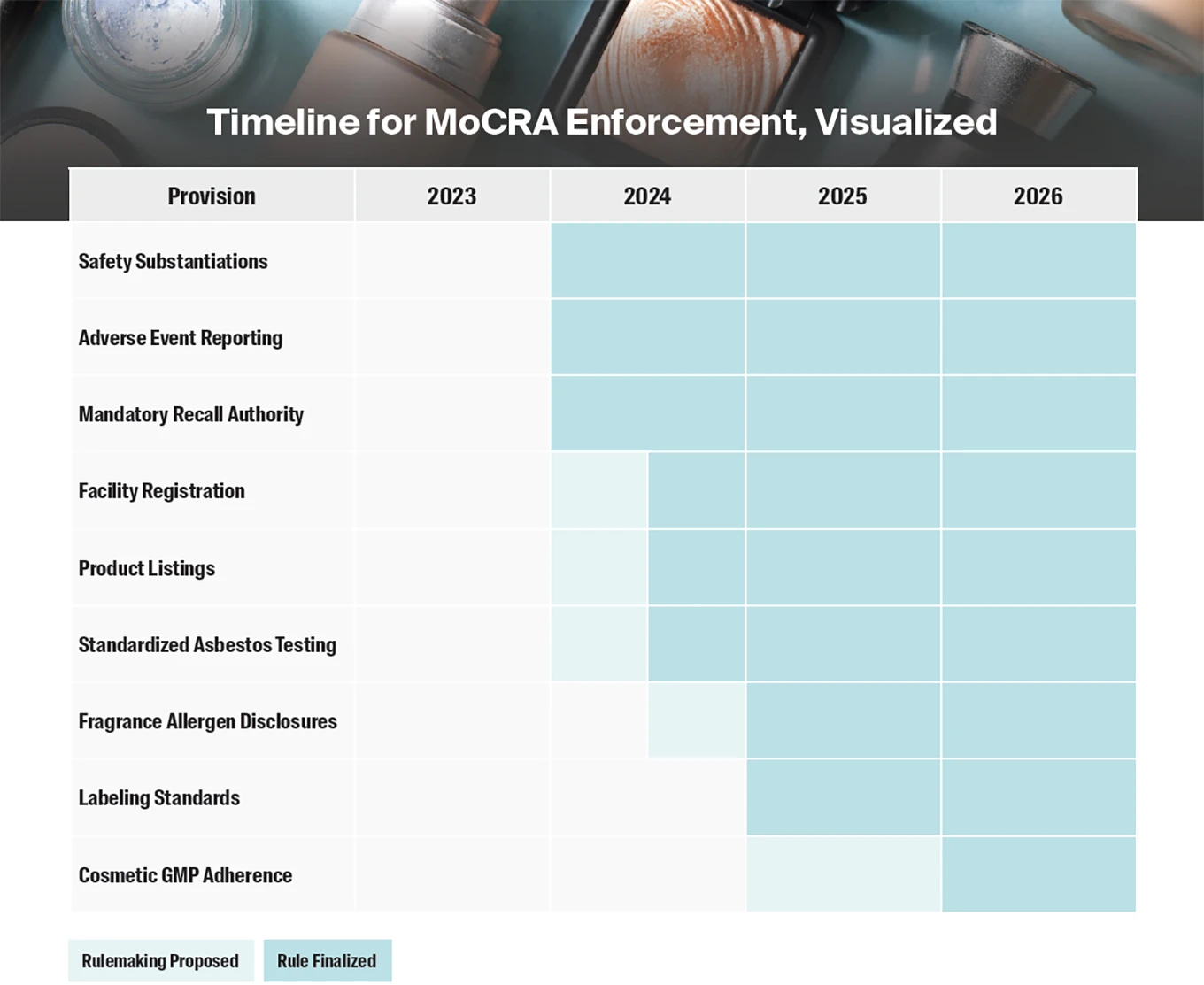
The new MoCRA requirements include:
Mandatory Adverse Event Reporting
Good Manufacturing Practices
Mandatory Business and Facility Registration
Cosmetic Product Listing
Product Safety Substantiation
Product Labeling Standards
FDA Records Access
FDA-Mandated Recalls
Standardized Asbestos Testing for Products Containing Talc
As a leading solutions provider committed to the industry, we’re here to help your business secure proven compliance with cosmetics regulations. Take a look at how our PLM for cosmetics companies can accelerate your compliance and much more.
explore the solution

How Cosmetics PLM Supports MoCRA Compliance
A cosmetics-specific product lifecycle management (PLM) system can offer robust features for managing your manufacturing processes while preparing your business for compliance with FDA cosmetic regulations, including:
Electronic Document Management (EDM) – create and store important reports, certificates and other documentation in a unified and fully digital repository.
Change Management – easily change product formulations with both single and mass component replacement functionalities.
Formulation Tool – allows for precise product formulation with the ability to integrate with universal databases like INCI.
Manufacturing Process Management – create standardized processes and assign them to specific products.
Packaging Development – tinker with your product packaging in accordance with updated labeling standards.
Compliance Module – provides ingredient statement creation, allergen screening and generation of important claims and certifications.
Aptean PLM for cosmetics is a purpose-built solution that can facilitate adherence to MoCRA regulations and help protect your business from the risks of noncompliance.
To empower your team to work more efficiently and cost-effectively while adhering to cosmetics regulations, read more about our industry-specific product lifecycle management solution, Aptean PLM Lascom Edition for cosmetics or get in touch with our team today.
Aptean Insights





Ready to start transforming your cosmetics regulatory compliance?
If you’re ready to ensure your cosmetics business is compliant with MoCRA, we’d love to help.




